Calcium lactate
Calcium lactate is a salt that consists of two lactate anions for each calcium cation (Ca2+). It is prepared commercially by the neutralization of lactic acid with calcium carbonate or calcium hydroxide. Approved by the FDA as a direct food substance affirmed as generally recognized as safe, calcium lactate is used as a firming agent, flavoring agent, leavening agent, stabilizer, and thickener. Calcium lactate is also found in daily dietary supplements as a source of calcium. It is also available in various hydrate forms, where calcium lactate pentahydrate is the most common.
CONTACT US- Overview
- Product Description
- Safety
- Packaging & Delivery
- Shipping & Payment FAQ
- Environmental
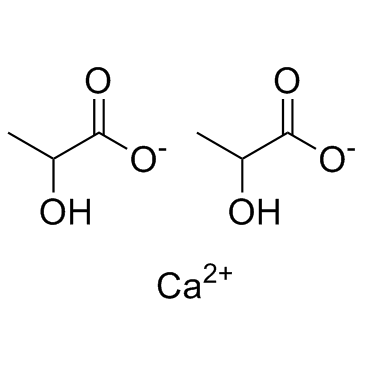
Synonyms: calcium lactate, 814-80-2, Calcium dilactate, calcium 2-hydroxypropanoate, Calphosan
CAS No: 814-80-2
Molecular formula: C3H5O2)2Ca·nH2O (n = 0 – 5) or C6H10CaO6
Molecular weight: 218.22 g/mol
Purity: 99%
Appearance: White to cream crystalline powder (est)
Melting point: 240 °C
Formulation: CALCIUM LACTATE USP; POWDER USP, TABLETS USP, 325 MG (42.25 MG CALCIUM), & 650 MG (84.5 MG CALCIUM)
SMILES: CC(C(=O)[O-])O.CC(C(=O)[O-])O.[Ca+2]
InChi Key: MKJXYGKVIBWPFZ-UHFFFAOYSA-L
Grade Standard: Food Grade, Medicine Grade
Brand name: Ikigai ™
Standard: USP, Ph. Eur., JP Etc
Storage: Store at room temperature
Shipping: Room Temperature (may vary elsewhere)
Stability (shelf life): AQ SOLN ARE PRONE TO BECOME MOLDY.
Origin: PRC
Both components of calcium lactate, calcium ion and lactic acid, play essential roles in the human body as a skeletal element an energy source, respectively [F59].
N/A
In aqueous environments such as the gastrointestinal (GI) tract, calcium lactate will dissociate into calcium cation and lactic acid anions, the conjugate base of lactic acid. Lactic acid is a naturally-occurring compound that serves as fuel or energy in mammals by acting as an ubiquitous intermediate in the metabolic pathways [F59]. Lactic acid diffuses through the muscles and is transported to the liver by the bloodstream to participate in gluconeogenesis [F59].
Calcium lactate is a salt that consists of two lactate anions for each calcium cation (Ca2+). It is prepared commercially by the neutralization of lactic acid with calcium carbonate or calcium hydroxide. Approved by the FDA as a direct food substance affirmed as generally recognized as safe, calcium lactate is used as a firming agent, flavoring agent, leavening agent, stabilizer, and thickener. Calcium lactate is also found in daily dietary supplements as a source of calcium. It is also available in various hydrate forms, where calcium lactate pentahydrate is the most common.
JECFA Functional Classes
Food Additives: ACIDITY_REGULATOR; FLOUR_TREATMENT_AGENT; YEAST_FOOD
EPA CPDat Chemical and Product Categories
| Category | Category Description | Categorization Type |
|---|---|---|
| Drug, dietary_supplement | Pharmaceutical related | CPCat Cassette |
| Drug, inactive_ingredient | Pharmaceutical related | CPCat Cassette |
| Facility, food_production | Related to food production (restaurants, catering, etc) | CPCat Cassette |
| Feed, animal | Related to animals (but non-veterinary) e.g., animal husbandry, farming of animals/animal production, raising of animals for food or fur, animal feed, products for household pets | CPCat Cassette |
| Food_additive | Includes spices, extracts, colorings, flavors, etc added to food for human consumption | CPCat Cassette |
Industry Processing Sectors
Agriculture, forestry, fishing and hunting
EPA TSCA Commercial Activity Status
Propanoic acid, 2-hydroxy-, calcium salt (2:1): ACTIVE
Commercial baking powder contains 2.5% calcium lactate.
Technological superiority and innovation
API manufacturing
| Pictogram(s) |
 |
|---|---|
| Signal |
Warning
|
| GHS Hazard Statements | Aggregated GHS information provided by 86 companies from 4 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria by 7 of 86 companies. For more detailed information, please visit ECHA C&L website Of the 3 notification(s) provided by 79 of 86 companies with hazard statement code(s): H319 (98.73%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
| Precautionary Statement Codes | P264, P280, P305+P351+P338, and P337+P313
(The corresponding statement to each P-code can be found at the GHS Classification page.) |
Disposal Methods
SRP: At the time of review, criteria for land treatment or burial (sanitary landfill) disposal practices are subject to significant revision. Prior to implementing land disposal of waste residue (including waste sludge), consult with environmental regulatory agencies for guidance on acceptable disposal practices.
FDA Requirements
Substance added directly to human food affirmed as generally recognized as safe (GRAS).
21 CFR 184.1207 (4/1/99)
Calcium lactate used as a general purpose food additive is generally recognized as safe when used in accordance with good manufacturing or feeding practice.
21 CFR 582.1207 (4/1/99)
CALCIUM LACTATE GIVEN ORALLY TO RABBITS @ 2.5 G/KG/DAY FOR 15 DAYS CAUSED HYPERPLASIA OF THE THYROID PARENCHYMA AND INCR RNA CONTENT IN THE THYROID MICROFOLLICLES AND FOLLICULAR EPITHELIUM.
Androstenedione’s production and administration as a drug to treat symptoms due to male andropause, dietary supplement, and athletic performance-enhancing drug may result in its release to the environment through various waste streams. Androstenedione is a naturally occurring androgen produced by the adrenal gland and converted to testosterone or estrone. It is produced naturally in humans during the production of testosterone and estrogen. It can be formed in soils via the metabolism of testosterone and estradiol occurring in poultry and livestock manures used as agricultural fertilizers. If released to air an extrapolated vapor pressure of 7.35X10-9 mm Hg at 20 °C indicates androstenedione will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase androstenedione will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals and with ozone; the half-lives for these reactions in the air are estimated to be 3.5 hours and 1 day respectively. Particulate-phase androstenedione will be removed from the atmosphere by wet and dry deposition. Androstenedione absorbs light at wavelengths >290 nm, and therefore, may be susceptible to direct photolysis. If released to soil, androstenedione is expected to have no mobility based upon a Koc of 6170. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry’s Law constant of 3.7X10-8 atm-cu m/mole. Androstenedione is not expected to volatilize from dry soil surfaces based upon its vapor pressure. Results of soil column studies using various soils and sands found that half-life of androstenedione ranged from 1.7 to 77 hours. If released into water, androstenedione is expected to adsorb to suspended solids and sediment based upon the Koc. Using OECD Guideline 301B (CO2 Evolution Test), androstenedione reached 79-95% degradation over a 28-day incubation period which classified the compound as readily biodegradable. Volatilization from water surfaces is not expected to be an important fate process based upon this compound’s estimated Henry’s Law constant. An estimated BCF of 30 suggests the potential for bioconcentration in aquatic organisms is low to moderate. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions. Androstenedione in aqueous solutions underwent direct photolysis half-lives ranging from 3.7 to 10.8 hours in direct sunlight. Occupational exposure to androstenedione may occur through dermal contact with this compound at workplaces where androstenedione is produced or used.
2000 Metric Ton/Metric Tons per Month
Packing:
Export worthy packing (1kg/Aluminium foil bag;25kg/drum) Or as per customers
request.
Ports: port of Hong Kong HKSAR
of PRC., port of Ningbo, PRC
or Any port in
China.
Delivery methods:
- by Air Freight & Cargo
- by Sea Freight & Cargo (Container shipping)
- by Rail Freight (train)
- by Road Freight
- by Intermodal freight transport
By couriers:
- SF Express
- STO Express
- DHL
- FEDEX
- EMS
- TNT
- UPS
- and almost any available courier service

Answer: Pro-forma invoice will be sent first after confirmation of the order, enclosed our payment information. Payment by T/T, Paypal, and others. All courier tracking numbers are provided upon shipping.
Answer: The MOQ for this API is 1kg.
Answer: We have shipping insurance. Our experienced team will do the customs documents, generally, it will have no trouble. And we will reship or refund for you if it is seized by our mistake.
Answer: Shipping lead time: About 2-5 days after payment confirmed. (Chinese holiday not included), We have long-term relations with shipping agents in main Chinese ports. Full service can be offered, including photos of every shipment, marks, and procedure of loading. General times are gor 1KG-100KG, Within 5-7 days by DHL, UPS, TNT, FEDEX, EMS Over 100KG. within 5-8days by air, 20-40days by sea. More information.
Answer: Different quantity has a different discount. Please feel free to contact us.
Answer: You can chat with us by Telegram, Email, Skype, Whatsapp, Facebook and other methods – just inquire, we will give a reply ASAP. Please feel free to send your quotations.
Answer: Our quality control will reduce the quality problem to near zero. If there is a real quality problem caused by us, we will send you free goods for replacement or refund your loss. We are competitive in API exporting, Pharmaceutical excipients, food supplements, dyestuff, veterinary API. Our team has 20 years of experience in this business. Good team for quality control, shipping, and documents.
U.S. funds preferably.
The negative impact of the production of pharmaceutical products on the natural environment is well known. However, this remains largely unregulated, meaning the extremely toxic impact it has on both animals and humans continues with no clear end in sight. As Ikigai Corporation Company Innovator, we are committed to expanding and improving our efforts to safeguard the environment. We accordingly established our environmental management system

Quality Management, Environmental Management and Occupational Safety
Policy
Ikigai Corporation Company is engaged in the production and distribution of
pharmaceutical substances and other chemical specialties. As a chemical and
pharmaceutical manufacturer, the Company is aware of the impacts of its activities on
the product quality, on the environment and the health of its employees, and undertakes
to control them, with the aim of constant improvement.
As standards for the implementation and maintenance of the Integrated Management System, the Company has chosen international standards ISO 9001 in the area of quality management (hereinafter called QMS), ISO 14001 for the management of environmental protection (hereinafter called EMS) and specification OHSAS 18000 for ensuring the occupational safety and health protection (hereinafter called SMS). The Integrated Management System applies to all fields of the Company’s activities.
Within this Policy, Ikigai Corporation Company undertakes to:
Constantly improve all of its activities
Executive commitment to continually improve energy efficiency across the entire
corporation, including clear processes and tracking systems to identify
opportunities
An empowered corporate energy director and energy team supported by sufficient human and
financial resources
A corporate energy policy that is accounted for at the top levels of the corporation
Aggressive, numeric energy goals that stretch performance targets to draw out creative
innovations for meeting them
Measurement and tracking of energy performance for all energy use, corporationwide,
including benchmarking facility performance nationally and globally with similar
companies, and a review system with accountability at all levels
Communication of the value of energy savings, importance of improving use of energy and
executive commitment by consistently recognizing accomplishments.
Abide by the relevant legislation and other regulations which apply to the Company or
which the Company has committed to observe; especially the requirements arisen from the
Act on Pharmaceuticals, the Decree on Good Manufacturing Practice and other guidelines
and directives and other requirements related to the occupational safety and the
environmental protection.
Constantly educate and train its employees and strongly encourage them to improve the
production quality, protect the environment and observe the occupational safety
principles; to design the training so as to motivate the employees performing their jobs
to prevent or reduce negative impacts of all activities on the environment.
Develop communication and cooperation with the public administration bodies,
professional public and other parties concerned with the environmental protection
issues. Ikigai Corporation Company is interested in holding open dialogue with the
employees and the public, in informing both internal and external stakeholders of the
impacts of all the Company’s activities on the environment and in responding to
justified suggestions and concerns.
The executive management expects that all the Company employees will accept an opinion
that observing the above mentioned principles and objectives is one of the most
fundamental duties of every employee in the Company.
Leave a Reply
Your email address will not be published. Required fields are marked *



