Effect of Fluoxymesterone Fish Feed Granule on Sex Reversal of the Hybrid, Thai Red Tilapia (Oreochromis niloticus Linn. x Oreochromis mossambicus Linn.)
J. MANOSROI1,2
, K. PETCHJUL3 and A. MANOSROI1,2
1Division of Pharmaceutical Science
Faculty of Pharmacy
Chiang Mai University, Chiang Mai
Thailand
2Pharmaceutical Cosmetic Raw Materials and Natural Products
Research and Development Center (PCRNC)
Institute for Science and Technology Research and Development (IST)
Chiang Mai University, Chiang Mai
Thailand
3Department of Fisheries
Rajamangala University of Technology
Kalasin Campus, Kalasin
Thailand
Abstract
Effects of an androgen, fluoxymesterone on sex reversal and gonadal development of Thai Red Tilapia, the hybrid of Oreochromis niloticus x O. mossambicus were investigated. Four days old fry were fed with feeds containing three different concentrations of fluoxymesterone (40, 60 and 80 mg•kg-1 fish feed) for 21 days. Fry fed with 40 mg•kg-1 fish feed of fluoxymesterone showed the highest percentage of males reversal of 96.10% (p < 0.05). Higher dose of fluoxymesterone at 60 and 80 mg•kg-1 fish feed increased the percentage of intersex and sterile fish. Higher growth rate of fish treated with fluoxymesterone in comparison to the control groups was observed. However, no significant differences in survival rate between the fluoxymesterone treated and the control groups were seen. No residual fluoxymesterone in 5 months old fish were detected. The morphological and histological studies demonstrated that fluoxymesterone can be used to control the gonadal development and phenotypic sex of the Thai Red Tilapia. This study suggested that fluoxymesterone can be used efficiently for sex reversal in the hybrid, Thai Red Tilapia.
Introduction
Sexuality of fish has great significance in aquaculture due to the differences in growth rate, survival rate, size, behavior pattern and breeding time.
In Tilapia, the culture of all male populations is important for higher growth rate and more uniformly sized fish can be obtained (Mair and Little 1991). This is because the fish exhibits sexually related dimorphic growth in which males grow and reach a larger ultimate size faster than the females (Guerrero 1975). In Thailand, Thai Red Tilapia is the hybrid between Oreochromis niloticus Linn. and Oreochromis mossambicus Linn. (Jarimopas 1988). It has been originally found from the Ubonratchathani Freshwater Fisheries Station, North-Eastern of Thailand in 1968 (Jarimopas 1988). Thai Red Tilapia is one of the most popular Tilapias because of its economic values.
The production of monosex populations has been successfully achieved by oral administration of natural or synthetic steroidal hormone to masculinize or feminize sexually undifferentiated fry (Pandian and Sheela 1995). This technique has been widely used. However, increasing consumer rejection to the use of hormone in food production has limited this application. Using hormones to alter the sex ratios of fish have been demonstrated in Eurasian perch, Perca fluviatilis (Rougeot et al. 2001), Black crappie, Pomoxis
nigromaculatus (Salam Al-ablani and Ronald Phelps 1997), European seabass, Dicentrarchus labrax (Chatain et al. 1999), Euryhaline tilapia, Oreochromis mossambicus (Benny Ron et al. 1995), Pikeperch, Stizostedion lucioperca (Demska-Zakes and Zakes 1997), Rainbow trout, Salmo gairdneri (Solar et al. 1984) and African catfish, Clarias gariepinus (Van Den Hurk et al. 1989). Sex hormones not only modify secondary sex characteristics, but also affect the gonads (Yamamoto 1951). Administration of feeds supplemented with synthetic androgenic hormones to sexually undifferentiated fry is one of the methods of choice for all-male sex reversal (Abucay and Mair 1997; Ohia and Takano 1996; Demska-Zakes and Zakes 1997). However, a synthetic androgenic hormone is based on testosterone, the principal male hormone synthesized in the testis, ovary and adrenal gland (Murad and Haynes 1985). Oral administration with synthetic hormone generally results in sex reversal, and the hormone is readily metabolized in several tilapiine species. (Goudie et al. 1986a; Goudie et al. 1986b; Curtis et al. 1991). However, high doses may result in gonadal growth reduction, gonadal intersexuality and feminization (Demska-Zakes and Zakes 1997). Alteration in spermatogenesis such as inhibition of spermiation, low fertility, and poor reproductive performance in several Teleostean species treated with synthetic androgen hormone have also been reported (George and Pandian 1996; Porter 1996). Other synthetic androgenic hormones such as 17a-methyltestosterone (Benny Ron et al. 1995), 17a-ethynyltestosterone (Guerrero 1975) and 17amethyldehydrotestosterone (Chatain et al. 1999) have been incorporated into the feed of fish for sex reversal. None of these androgens can be considered the best for tilapia sex reversal. The range of dose and treatment protocols make it difficult to compare these hormones. One perspective to evaluate these androgens is based on the production cost of a given number of fish. Phelps et al (1992) discussed how fluoxymesterone became more expensive than 17a-methyltestosterone whereas the effective dose was lower, thus compensating for the cost difference. Selection of androgen to be incorporated into the feed should be based on availability, costs, government regulations, and ecological impact. Fluoxymesterone and 17a-methyltestosterone can be harmful by inhalation, ingestion, or skin absorption and may cause irritation. Preparation of these hormones in fish feed granular form will minimize the distribution of feeds and hormone to the environment that will be hazardous to human. In granular form, the feed can also reduce the loss of feeds and hormone due to the physical properties of granules which is suitable for the eating behavior of the fry.
In the present work, fish feeds containing fluoxymesterone was formulated in granular form and the effects of fluoxymesterone concentrations in fish feeds on sex reversal and gonadal development of the hybrid, Thai Red Tilapia were investigated.
Materials and Methods
Fry production
Male and female hybrid, Thai Red Tilapia (Oreochromis niloticus x O. mossambicus) with the average weight of 290 ±5.50 g and length of 23.20±2.30 cm were reared in rectangular hapas constructed with the 1.60 mm mesh net in the size of 5 m3. The hapas were designed to allow fish to be crowded at one end for convenient collection. A total of 80 brooders were reared in each hapa at a ratio of 1:3 male/female. Fish were spawned naturally in the hapas. After 14 days of spawning, brooders and the sec fry were removed, and the females were taken out for eggs and fry examination. Eggs were collected and then hatched in aquaria. Fry were reared in fine mesh hapas installed in earthen ponds. Dissolved oxygen contents (5.50 -8.00 mg•l-1), pH (7.00 – 7.80), ammonia contents (0.10 – 0.45 mg•l-1) and the average temperature (25 ± 1.00°C) were maintained and measured weekly throughout the experiments. Fish rearing was performed at Department of Fisheries Technology, Maejo University, Chiang Mai, Thailand.
Fish feed preparation
Fish feed granules were prepared by wet granulation method using 2.50% corn starch solution as binder. Corn starch solution was prepared by adding 80 ml of boiling water to 2.50 g of corn starch powder and adjusted to 100 ml. A 100 g of fish meal (commercial powdered fish which passed through sieve No. 40) were mixed with various concentrations of fluoxymesterone (Sigma Co., USA) solution in 95% ethyl alcohol and 90.10 ml of 2.50% corn starch solution for 5 minutes to get a wet mass. The wet mass was then passed through sieve No. 8 and dried at 50ºC for 18 hours. The final concentration of fluoxymesterone were 40, 60 and 80 mg•kg-1 fish feed, respectively. The dried granules were passed through sieve No 20. The control feed was prepared in the same manner
but without the hormone. The fish feed granules were kept in a tight container at 4ºC prior to use.
Hormonal treatments
The 4-day old fish after hatching were divided into 5 treatments of 300 fish per treatment. Each treatment was divided into 3 groups. In treatments1 and 2, the fish were fed with commercial fish meal and fish granule without hormone, respectively. For treatments 3 to 5, the fish were fed with fish granule containing hormone at 40, 60 and 80 mg•kg-1 of fish feed, respectively. Fry were fed daily at 08.00 AM, 10.00 AM, 14.00 PM and 16.00 PM at 10% of body weight for 21 days. After 21 days, fry were transferred and
grown in a 1 m3 fine mesh hapa installed in earthen ponds (25 x 40 x1.50 m3). Then, all fish were fed with normal feed. Twenty fish from each group were taken randomly, weighed and measured (the length). Growth and survival rates were determined twice a month for 5 months. The residual fluoxymesterone in fish, 5 months old, were detected by HPLC (Highperformance liquid chromatograms).
Histological examination of gonads
At the end of the experiments, 20 fish were taken randomly from each hapa for morphological and histological examinations of gonads. Gonadal tissues were dissected, fixed in Bouin’s fixative and embedded in paraffin wax.
Tissue cross-sections (4-6 µm thin) were prepared using a microtome (Microtome Cryostat, I.E.C.). The sections were stained with haematoxylin and eosin and mounted on glass microslides. The slides were examined and photographed using a stereomicroscope (Nikon Optiphot, Nikon Corp., Tokyo, Japan). The presence of spermatogonia and ovocells in the gonads were used as an indicator of males and females respectively. Intersex gonads contained both oogenic and spermatogenic tissue. Sterile gonads contained large amounts of connective tissue with numerous vessels. Quantitative assay of fluoxymesterone Fluoxymesterone from Sigma Co., USA were used as reference standards. The fish samples (n = 20 per group) were used. A 10 gm of fish tissue was homogenized and extracted with 20 ml CH3OH. The extracts were centrifuged at 3,000 rpm. Subsequently, 10 ml of the filtered supernatant was assayed by HPLC (HP 1100, Vectra XM series 4, Hewlett Packard, USA). The column used was a 5 mm, 250 x 4.6 mm Selectosil C 18 (Phenomenex, USA) at a flow rate of 1 ml•min and the wavelength at 254 nm. The mobile phase was composed of methanol and water at a ratio of 70:30 v/v.
Statistical analysis of data
One way ANOVA (a = 0.05) and Duncan’s multiple range test were used for the statistical analysis.
Results
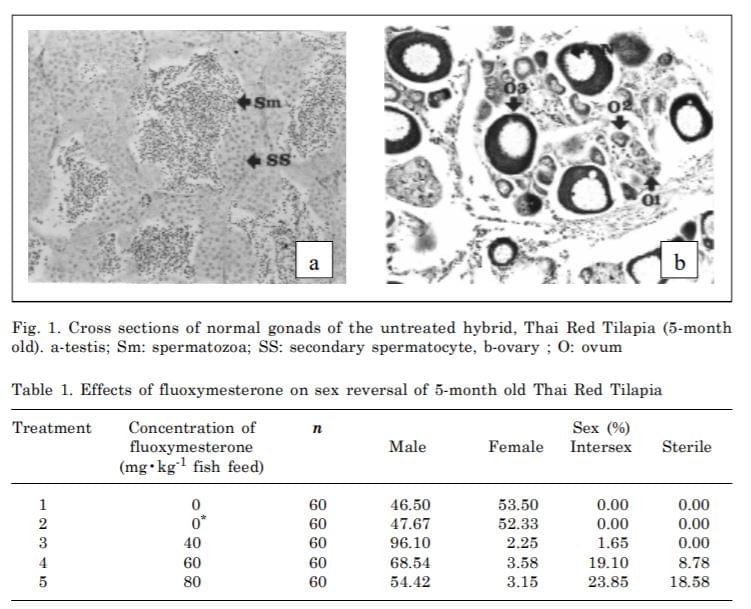
In the control group, gonadal development was normal both in testis and ovaries. The spermatogonia and ovocells in the gonads were found in normal males and females, respectively. The males had relatively smaller lobate gonads containing primordial germ cells (PGC), gonocytes and spermatogonia and appeared to be normal both in morphology and histology. (Fig. 1a). Ovaries of the females have medially located ovocells containing oogonia, meiotic oocytes and oocytes at the previtellogenesis stage (Fig. 1b).
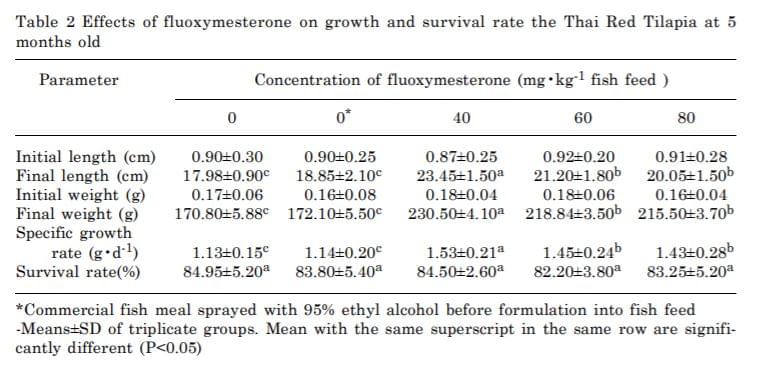
From gonadal histological examination the sex percentage of male to female range from 46.50 to 53.50% (Table 1).
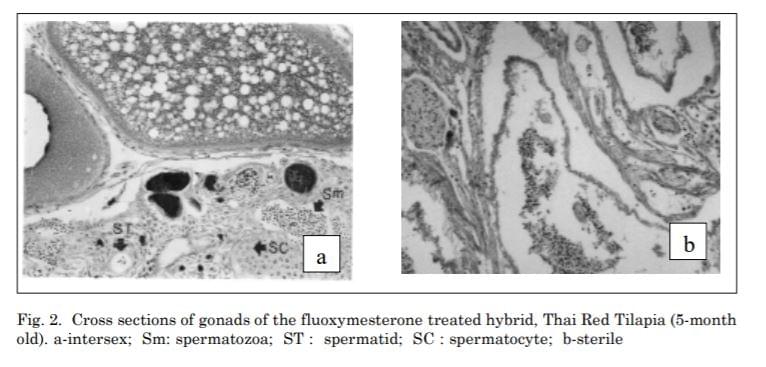
The groups treated with fluoxymesterone at 40 mg•kg-1 fish feed showed three types of gonads which were 96.10% males, 2.25 % females and1.65% intersex. Histological examination revealed that ovaries and testis in the treated group were the same as the control groups. However, abnormal gonads in intersex containing numerous small spaces appeared (Fig. 2a).
At a dose of 60 mg•kg-1 fish feed of fluoxymesterone, 68.54% male, 3.58% female, 19.10% intersex and 8.78% sterile gonads were observed. Intersex gonads contained both oogenic and spermatogenic tissue. At a higher dose of 80 mg•kg-1 fish feed, not only higher percentage of intersex (23.85%) was obtained, but also higher percentage of sterile gonads of 18.58% with
large amounts of connective tissue containing numerous vessels were observed as well (Fig. 2b). Lobes with male germ cells were also sporadically found in these tissues.
Mean length, weight and survival rate of all treatments were presented in table 2. The growth rates in the fluoxymesterone-treated groups were higher than the control groups (P < 0.05) with the specific growth rate ranging from 1.13 ± 0.15 to 1.53 ± 0.21. No significant differences in survival rate (ranging from 82.20±3.80 to 84.95 ± 5.20) between treated and the control group (P < 0.05) were observed. The remaining fluoxymeoterone determined by HPLC in the 5 months old fish of al treated groups were not detected (data not shown).
Discussion
Thai Red Tilapia has sexual growth dimorphism in which males grow significantly faster than females. Currently, fish feed formulation for sex reversal of Tilapia is prepared in powdered form by mixing male hormone with fish meal. Preparation of fish feed granules with corn starch had lower hormone loss in water and gave advantage characteristices of better disintegration and distribution in water. Fluoxymesterone has never been used for the induction of sex reversion in Tilapia. Using fish feeds powder containing hormone can be harmful if swallowed, inhaled or absorbed through skin and may cause irritation. In this study, preparation of fish feeds granules with corn starch as a binding agent can minimize these disadvantages by lowering hormone loss due to the excess of powdered feed and dusty distribution.
The advantage of using hormone in granular form is the convenience and better disintegration and distribution in water. Treatment of fish fry for 21 days with fluoxymesterone at 40 mg•kg-1 of fish feed was the most effective dose in inducing masculinization of the hybrid, Thai Red Tilapia with a yield of 96.10% male. This hormone concentration is the same as that used for Eurasian perch, Perca fluviatilis of 17a-methyltestosterone (Rougeot et al. 2001) and for Black crappie, Pomoxis nigromaculatus of 17a-methyltestosterone (Salam Al-ablani and Ronald Phelps 1997). However, this dose is lower than that was used for the induction of sex reversal in common carp, Cyprinus carpio (100 mg of 17amethyltestosterone•kg-1 fish feed; Gomelsky et al. 1994), but higher than that used in European seabass, Dicentrarchus labrax (0.5-5 mg of 17amethyldehydrotestosterone kg-1 fish feed; Chatain et al. 1999) or the Euryhaline tilapia, Oreochromis mossambicus (10 mg of 17a-methyltestosterone kg-1 fish feed; Benny Ron et al. 1995). Higher doses (60 and 80 mg•kg-1 fish feed) of this hormone gave lower yields of male to female. In our experiment, fluoxymesterone at 40 mg•kg-1 fish feed appeared to be the lowest and optimum doses comparing with the other male hormones used.
Generally, effectiveness of androgen treatment depends on doses. Nevertheless, numerous studies of hormones on gonadal development have shown inhibitory effects of the drug at high dosages (Van Den Hurk and Slof 1981). At high dosages, some of the treated individuals become sterile. Intersex and sterility occurrence among the steroid-treated fish suggest two patterns. In the first pattern, intersex appears at a sub-optimum intensity of the treatment. For the second pattern, intersex and sterile individuals simultaneously occur among the fish treated at super-optimal doses (Pandian and Sheela 1995). Moreover, paradoxical feminizing effects of high dosages of androgens were found in Pikeperch, Stizostedion lucioperca (Demska-Zakes and Zakes 1997), Rainbow trout, Salmo gairdneri (Solar et al. 1984), African catfish,
Clarias gariepinus (Van Den Hurk et al. 1989) and species cichlidae (Nakamura 1975). The optimal concentration of fluoxymesterone for the sex reversal of Thai Red Tilapia fry was 40 mg•kg-1 fish feed. A similar result was also reported in Pikeperch, Stizostedion lucioperca using 17a-methyltestosterone at 30 mg•kg-1 fish feed (Demska-Zakes and Zakes 1997). Effects of androgenic hormone on the development of sex-reversal gonads can be divided into 2 stages. The first stage is the development of gonadal germinal tissue. The second stage is the repopulation of the testicular tissue by gonocytes. Hackmann and Reinboth (1973) concluded that administration of exogenous hormone causes complete or partial degeneration of female gonocytes. Higher growth rates of hormone-treated fish were also observed. However, there was no significant difference in survival rate in all treatments. These results conform to what was reported in Pikeperch, Stizostedion lucioperca (Demska-Zakes and Zakes 1997).
Conclusion
The most significant masculinization effect of fluoxymesterone was observed at 40 mg•kg-1 fish feed. All hormone-treated fish showed higher growth rate than the control groups. The effective dose of fluoxymesterone was lower than 17a-methyltestosterone, thus reducing fry production cost. Selection of androgen for sex reversal to be incorporated into fish feed should be based on costs, effectiveness and human/environmental safety. In addition, no fluoxymesterone in 5-month old fish was detected which confirmed residual free fluoxymesterone fish. The information obtained from this study provides a useful information for sex reversal and commercial scale production of Thai Red Tilapia by using fluoxymesterone.
Acknowledgments
This work was supported by grants from the Graduate School, Chiang Mai University, Chiang Mai, Thailand and Rajamangala University of Technology, Kalasin Campus, Kalasin, Thailand.
References
Abucay, J.S. and G.C. Mair. 1997. Hormonal sex reversal of tilapias : implications of hormone treatment application in close water system. Journal of Aquaculture Research 28: 841-845.
Benny, R., K. Steve, Shimoda., K. George, E. Iwama and G. Grau. 1995. Relationships among ration, salinity, 17a methyltestosterone and growth in the euryhaline tilapia, Oreochromis mossambicus. Aquaculture 135: 185-193.
Chatain, B., E. Saillant, and S. Peruzzi. 1999. Production of monosex male populations of Europian seabass, Dicentrarchus labrax L. by use of the synthetic androgen 17 amethyldehydrotestosterone. Aquaculture 178: 225-234
Curtis, L.R., F.T. Diren, M.D. Hurley, W.K. Seim and R.A. Tobb.1991. Disposition and elimination of 17 a-methyltestosterone in Nile tilapia (Oreochromis niloticus). Aquaculture 99: 193-201.
Demska-Zakes, K. and Z. Zakes. 1997. Effect of 17a-methyltestosterone on gonadal differentiation in pikeperch, Stizostedion lucioperca L. Journal of Aquaculture Research 28 : 59-63.
George, T. and T.J. Pandian. 1996. Hormonal induction of sex reversal and progeny testing in the Zebra cichlid, Cichlasoma nigrofasciatum. Journal of Experimental Zoology 275: 374-382.
Gomelsky, B.I., N.B. Cherfas, Y. Peretz, N. Ben-Dom and G. Hulata. 1994. Hormonal sex inversion in the Common carp (Cyprinus carpio L.). Aquaculture 126: 265-270.
Goudie, C.A., W.L. Shelton and N.C. Parker. 1986a. Tissue distribution and elimination of radiolabelled methyltestosterone fed to adult blue tilapia. Aquaculture 58: 227-240.
Goudie, C.A.., W.L. Shelton and N.C. Parker. 1986b. Tissue distribution and elimination of radiolabelled methyltestosterone fed to sexually undifferentiated blue tilapia. Aquaculture 58: 215-226.
Guerrero III, R.D. 1975. Use of androgens for the production of all male Tilapia aurea (Steindachner). Transactions of the American Fisheries Society 104: 342-348.
Hackmann, E. and R. Reinboth. 1973. Delimitation of the critical stage of hormone-influenced sex differentiation in Hemihaplochromis multicolor (Higendorf) (Cichlidae). Journal of General Comparative Endrocrinology 22: 42-53.
Jarimopas, P. 1988. Thai Red Tilapia. Journal of Thai Fisheries Gazette. 41 : 41-43. (In Thai).
Mair, G.C. and D.C. Little. 1991. Population control in farmed tilapia. NAGA, ICLARM Q. 14: 8-13.
Murad F, and R.C. Haynes. 1985. Androgen. In. Ed: Goodman Gilman A, Goodman L S, Roll T W, Murad F. The Pharmacological Basis of Therapeutics, 7th edition, Macmillan, NewYork: 1440-1458.
Nakamura, Y. 1975. Dosage-dependent changes in the effect of oral administration of methyltestosterone on gonadal differentiation in Tilapia mossambicus. Bulletin of the Faculty of fisheries, Hokkaido University 26: 99-108.
Ohia, H. and K. Takano. 1996. Testicular maturation induced by methyltestosterone in elvers of the Japaness eel, Anguilla japonica. Journal of Fisheries Science 62: 99-991.
Pandian, T.J. and S.G. Sheela. 1995. Hormonal induction of sex reversal in fish. Aquaculture 138: 1-22. Phelps, R.P., W. Cole and T. Katz. 1992. Effect of fluoxymesterone on sex ratio and growth of Nile tilapia Oreochromis niloticus (L.) Aquaculture and Fisheries Management 23: 405-410.
Porter, M.D. 1996. Effects of methyltestosterone on largemouth bass, Micropterus salmoldes. Journal of Applied Aquaculture 6: 39-46.
Rougeot, C., B. Jacobs, P. Kestemont and C. Melard. 2001. Sex control and sex determinism study in Eurasian perch, Perca fluviatilis, by use of hormonally sex-reversed male breeders. Aquaculture (In prees).
Salam, A., Al-ablani and Ronald, P. Phelps. 1997. Sex reversal in Black crappie Pomoxis nigromaculatus: effect of oral administration of 17 a-methyltestosterone on two age classes. Aquaculture 158: 155-165.
Solar, I.I., E.M. Donaldson and G.A. Hunter. 1984. Optimalization of treatment regimes for controlled sex differentiation and sterilization in wild rainbow trout (Salmo gairdneri Richardson) by oral administration of 17 a-methyltestosterone. Aquaculture 42: 129-139.
Van Den Hurk, R. and G.A. Slof. 1981. A morphological and experimental study of gonadal sex differentiation in the rainbow trout, Salmo gairdneri. Journal of Cell and tissue Research 218: 487-497.
Van Den Hurk, R., C.J.J. Richter and J. Janssen-Dommerholt. 1989. Effect of 17 a-methyltestosterone and 11 b hydroxyandrostenedion on gonal differentiation in the African catfish, Clarias gariepinus. Aquaculture 83: 179-191.
Varadaraj, K. 1990. Endocrine and genetic studies on sex regulation in tilapia. Ph.D. Thesis, Madurai Kamraj University, Madurai.
Yamamoto, T. O. 1951. Artificial induction of sex-reversal in genotypic females of the medaka ( Oryzias latipes). Journal of Experimental Zoology 123: 571-594.
Source:
https://www.asianfisheriessociety.org/publication/downloadfile.php?id=601&file=Y0dSbUx6QXhORFF6TnpVd01ERXpOVFU1TURFMk1UWXVjR1Jt&dldname=Effect%20of%20Fluoxymesterone%20Fish%20Feed%20Granule%20on%20Sex%20Reversal%20of%20the%20Hybrid,%20Thai%20Red%20Tilapia%20(Oreochromis%20niloticus%20Linn.%20x%20Oreochromismossambicus%20Linn.).pdf
