Estradiol Valerate
Application: For R&D Purpose.
WARNING! This product is not for human or veterinary use.
Need larger quantities for your development, manufacturing or research applications? Contact us.
CONTACT US- Overview
- Product Description
- Safety
- Packaging & Delivery
- Shipping & Payment FAQ
- Environmental
Estradiol valerate is a novel estrogen, whose structure is similar to that of 17β-estradiol and which is rapidly metabolized to 17β-estradiol and valeric acid.
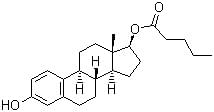
Synonyms: Estradiol 17 beta, Estradiol, (8 alpha,17 beta)-(+-)-Isomer;
(1S,11S,14S,15S,10R
CAS No: 979-32-8
Molecular formula: C23H32O3
Molecular weight: 356.5 g/mol
Purity: 99%
Appearance: Off-white to white powder (colour may vary)
Melting point: 144°C
Boiling Point: 486.2±45.0 °C (at 760 mmHg)
Flash Point: 191.1±21.5 °C
Formulation: A neat solid powder
SMILES: CCCCC(=O)OC1CCC2C1(CCC3C2CCC4=C3C=CC(=C4)O)C
InChi Key: RSEPBGGWRJCQGY-RBRWEJTLSA-N
Grade Standard: Pharmaceutical-grade
Brand name: Ikigai ™
Standard: USP, Ph. Eur., JP
Etc
Storage: -20°C
Shipping: Room Temperature (may vary elsewhere)
Stability (shelf life): ≥ 3 years
Origin: PRC
The suggested storage temperature is at -20℃, but the package is usually shipped at room
temperature.
1. By “the suggested storage temperature is at -20℃”, it means need to keep the left
compound at -20℃ after the package was opened and the API could not be used for a
prolonged period of time.
2. Most of our APIs are in dry powder and have very good stability, so it is safe during
the transition at room temperature and it could last for 1-2 weeks and even more.
3. For some specific sensitive compounds, we will ship them with dry ice or blue ice
bags.
EPA CPDat Chemical and Product Categories
| Category | Category Description | Categorization Type |
|---|---|---|
| Drug | Drug product, or related to the manufacturing of drugs; modified by veterinary, animal, or pet if indicated by source | CPCat Cassette |
| Drug, adjuvant | Pharmaceutical related | CPCat Cassette |
| Drug, dea_abuse_concern | Pharmaceutical related | CPCat Cassette |
| Drug, orphan | Pharmaceutical related | CPCat Cassette |
| Food | Food for human consumption, does not include food additives (see food_additive); also includes manufacture of food, facilities related to food (with appropriate modifiers) | CPCat Cassette |
The drug or other substance has a currently accepted medical use in treatment.
Technological superiority and innovation
API manufacturing
| Pictogram(s) |
  |
|---|---|
| Signal |
Danger
|
| GHS Hazard Statements | Aggregated GHS information provided by 84 companies from 9 notifications to
the ECHA C&L Inventory. Each notification may be associated with
multiple companies.
H302 (21.43%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (20.24%): Harmful in contact with skin [Warning Acute toxicity, dermal] H332 (20.24%): Harmful if inhaled [Warning Acute toxicity, inhalation] H351 (50%): Suspected of causing cancer [Warning Carcinogenicity] H360 (96.43%): May damage fertility or the unborn child [Danger Reproductive toxicity] H362 (28.57%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H413 (29.76%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
| Precautionary Statement Codes | P201, P202, P260, P261, P263, P264, P270, P271, P273, P280, P281, P301+P312,
P302+P352, P304+P312, P304+P340, P308+P313, P312, P322, P330, P363, P405,
and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.) |
Disposal Methods
SRP: Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state-licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Personal precautions, protective equipment and emergency procedures: Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. Environmental precautions: Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided. Methods and materials for containment and cleaning up: Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal.
Skin TDLo (woman): 4 µg/kg (36h); Subcutaneous TDLo (rat): 5 µg/kg; Subcutaneous TDLo (mouse): 1 µg/kg.
2000 Metric Ton/Metric Tons per Month
Packing:
Export worthy packing (1kg/Aluminium foil bag;25kg/drum) Or as per customers
request.
Ports: port of Hong Kong HKSAR
of PRC., port of Ningbo, PRC
or Any port in
China.
Delivery methods:
- by Air Freight & Cargo
- by Sea Freight & Cargo (Container shipping)
- by Rail Freight (train)
- by Road Freight
- by Intermodal freight transport
By couriers:
- SF Express
- STO Express
- DHL
- FEDEX
- EMS
- TNT
- UPS
- and almost any available courier service

Answer: Pro-forma invoice will be sent first after confirmation of the order, enclosed our payment information. Payment by T/T, Paypal, and others. All courier tracking numbers are provided upon shipping.
Answer: The MOQ for this API is 1kg.
Answer: We have shipping insurance. Our experienced team will do the customs documents, generally, it will have no trouble. And we will reship or refund for you if it is seized by our mistake.
Answer: Shipping lead time: About 2-5 days after payment confirmed. (Chinese holiday not included), We have long-term relations with shipping agents in main Chinese ports. Full service can be offered, including photos of every shipment, marks, and procedure of loading. General times are gor 1KG-100KG, Within 5-7 days by DHL, UPS, TNT, FEDEX, EMS Over 100KG. within 5-8days by air, 20-40days by sea. More information.
Answer: Different quantity has a different discount. Please feel free to contact us.
Answer: You can chat with us by Telegram, Email, Skype, Whatsapp, Facebook and other methods – just inquire, we will give a reply ASAP. Please feel free to send your quotations.
Answer: Our quality control will reduce the quality problem to near zero. If there is a real quality problem caused by us, we will send you free goods for replacement or refund your loss. We are competitive in API exporting, Pharmaceutical excipients, food supplements, dyestuff, veterinary API. Our team has 20 years of experience in this business. Good team for quality control, shipping, and documents.
U.S. funds preferably.
The negative impact of the production of pharmaceutical products on the natural environment is well known. However, this remains largely unregulated, meaning the extremely toxic impact it has on both animals and humans continues with no clear end in sight. As Ikigai Corporation Company Innovator, we are committed to expanding and improving our efforts to safeguard the environment. We accordingly established our environmental management system

Quality Management, Environmental Management and Occupational Safety
Policy
Ikigai Corporation Company is engaged in the production and distribution of
pharmaceutical substances and other chemical specialties. As a chemical and
pharmaceutical manufacturer, the Company is aware of the impacts of its activities on
the product quality, on the environment and the health of its employees, and undertakes
to control them, with the aim of constant improvement.
As standards for the implementation and maintenance of the Integrated Management System, the Company has chosen international standards ISO 9001 in the area of quality management (hereinafter called QMS), ISO 14001 for the management of environmental protection (hereinafter called EMS) and specification OHSAS 18000 for ensuring the occupational safety and health protection (hereinafter called SMS). The Integrated Management System applies to all fields of the Company’s activities.
Within this Policy, Ikigai Corporation Company undertakes to:
Constantly improve all of its activities
Executive commitment to continually improve energy efficiency across the entire
corporation, including clear processes and tracking systems to identify
opportunities
An empowered corporate energy director and energy team supported by sufficient human and
financial resources
A corporate energy policy that is accounted for at the top levels of the corporation
Aggressive, numeric energy goals that stretch performance targets to draw out creative
innovations for meeting them
Measurement and tracking of energy performance for all energy use, corporationwide,
including benchmarking facility performance nationally and globally with similar
companies, and a review system with accountability at all levels
Communication of the value of energy savings, importance of improving use of energy and
executive commitment by consistently recognizing accomplishments.
Abide by the relevant legislation and other regulations which apply to the Company or
which the Company has committed to observe; especially the requirements arisen from the
Act on Pharmaceuticals, the Decree on Good Manufacturing Practice and other guidelines
and directives and other requirements related to the occupational safety and the
environmental protection.
Constantly educate and train its employees and strongly encourage them to improve the
production quality, protect the environment and observe the occupational safety
principles; to design the training so as to motivate the employees performing their jobs
to prevent or reduce negative impacts of all activities on the environment.
Develop communication and cooperation with the public administration bodies,
professional public and other parties concerned with the environmental protection
issues. Ikigai Corporation Company is interested in holding open dialogue with the
employees and the public, in informing both internal and external stakeholders of the
impacts of all the Company’s activities on the environment and in responding to
justified suggestions and concerns.
The executive management expects that all the Company employees will accept an opinion
that observing the above mentioned principles and objectives is one of the most
fundamental duties of every employee in the Company.
Leave a Reply
Your email address will not be published. Required fields are marked *



