Paul E. Goss, M.D., Ph.D., James N. Ingle, M.D., José E. Alés-Martínez, M.D., Ph.D., Angela M. Cheung, M.D., Ph.D., Rowan T. Chlebowski, M.D., Ph.D., Jean Wactawski-Wende, Ph.D., Anne McTiernan, M.D., John Robbins, M.D., Karen C. Johnson, M.D., M.P.H., Lisa W. Martin, M.D., Eric Winquist, M.D., Gloria E. Sarto, M.D.,
ABSTRACT
BACKGROUND
Tamoxifen and raloxifene have limited patient acceptance for primary prevention of breast cancer. Aromatase inhibitors prevent more contralateral breast cancers and cause fewer side effects than tamoxifen in patients with early-stage breast cancer.
METHODS
In a randomized, placebo-controlled, double-blind trial of exemestane designed to detect a 65% relative reduction in invasive breast cancer, eligible postmenopausal women 35 years of age or older had at least one of the following risk factors: 60 years of age or older; Gail 5-year risk score greater than 1.66% (chances in 100 of invasive breast cancer developing within 5 years); prior atypical ductal or lobular hyperplasia or lobular carcinoma in situ; or ductal carcinoma in situ with mastectomy. Toxic effects and health-related and menopause-specific qualities of life were measured.
RESULTS
A total of 4560 women for whom the median age was 62.5 years and the median Gail risk score was 2.3% were randomly assigned to either exemestane or placebo. At a median follow-up of 35 months, 11 invasive breast cancers were detected in those given exemestane and in 32 of those given placebo, with a 65% relative reduction in the annual incidence of invasive breast cancer (0.19% vs. 0.55%; hazard ratio, 0.35; 95% confidence interval [CI], 0.18 to 0.70; P=0.002). The annual incidence of invasive plus noninvasive (ductal carcinoma in situ) breast cancers was 0.35% on exemestane and 0.77% on placebo (hazard ratio, 0.47; 95% CI, 0.27 to 0.79; P=0.004). Adverse events occurred in 88% of the exemestane group and 85% of the placebo group (P=0.003), with no significant differences between the two groups in terms of skeletal fractures, cardiovascular events, other cancers, or treatment-related deaths. Minimal quality-of-life differences were observed.
CONCLUSIONS
Exemestane significantly reduced invasive breast cancers in postmenopausal women who were at moderately increased risk for breast cancer. During a median follow-up period of 3 years, exemestane was associated with no serious toxic effects and only minimal changes in health-related quality of life. (Funded by Pfizer and others; NCIC CTG MAP.3 ClinicalTrials.gov number, NCT00083174. opens in new tab.)
Estrogens contribute to normal breast development but can also promote breast cancer in preclinical models and in women with high circulating plasma estrogen levels.1-4 To date, chemoprevention of breast cancer has focused on the selective estrogen-receptor modulators (SERMs) tamoxifen and raloxifene, which exert antiestrogenic effects on the breast, as well as agonist or antagonist effects on other organs. In the National Surgical Adjuvant Breast and Bowel Project P-1 trial, tamoxifen significantly reduced the number of invasive breast cancers, by 49% (P<0.001) as compared with placebo.5 A meta-analysis of trials comparing tamoxifen with placebo showed that tamoxifen reduced the incidence of breast cancer by 38% with no effect on mortality.6 On the basis of these collective data on tamoxifen, the estimated number needed to treat to prevent one breast cancer after 5 years is about 95 and is reduced to 56 after 10 years.7 Similar risk reductions occur with raloxifene.8-10 Tamoxifen increases the risks of endometrial cancers and venous thromboembolism; raloxifene does not increase the risk of endometrial cancers but does cause similar toxic effects.
The acceptance of tamoxifen or raloxifene for reducing the risk of breast cancer has been poor, in part because they are both associated with rare but serious toxic effects.11-13 Of the approximately 2 million U.S. women who could potentially benefit from treatment with tamoxifen, only 4% of those at increased risk for breast cancer and only 0.08% of all U.S. women 40 to 79 years of age have accepted the use of this drug for chemoprevention.13-15 A 2002 expert assessment concluded that tamoxifen lacks overall health benefits and recommended that future trials be conducted with placebo controls.16 Novel, less toxic interventions are needed that will reduce the threshold of risk yet provide a net benefit.17
Aromatase inhibitors profoundly suppress estrogen levels in postmenopausal women and inhibit the development of breast cancer in laboratory models.18-21 In early trials of breast cancer therapy, both nonsteroidal and steroidal aromatase inhibitors reduced contralateral primary breast cancers more than did tamoxifen; after 5 years of tamoxifen therapy, letrozole resulted in a further reduction of 46%, as compared with placebo.22-27 Preclinical models and clinical studies suggest that because of exemestane’s antiestrogenic effects, such as those on bone resorption due to this drug’s mild androgenic activity, it is a good candidate for study in a breast-cancer prevention trial.28-30
Methods
STUDY DESIGN
The NCIC Clinical Trials Group Mammary Prevention.3 trial (NCIC CTG MAP.3) is an international, randomized, double-blind, placebo-controlled trial conducted in Canada, the United States, Spain, and France. The trial was approved by the health regulatory authorities and institutional review boards at the participating centers, and enrollment began in September 2004. After stratification according to current use of low-dose aspirin (≤100 mg per day) (yes or no) and Gail risk score (for calculation of this score, see www.cancer.gov/bcrisktool. opens in new tab and the Supplementary Appendix, available with the full text of this article at NEJM.org) (≤2.0% or >2.0%), subjects were randomly assigned to one of three treatment groups with the use of a dynamic minimization algorithm: 25 mg of exemestane plus placebo, 25 mg of exemestane plus celecoxib, or placebo plus placebo pills, administered daily after a morning meal.31,32 After 31 patients were enrolled, 10 patients discontinued treatment with celecoxib because of concern for cardiovascular safety.33 Before enrollment and during the study, written informed consent and reconsent in all the participating countries included counseling about the risks and benefits of treatment with tamoxifen and raloxifene. The trial was event-driven, with a planned maximum duration of therapy of 5 years or until a breast event, a neoplastic disease, or a cardiovascular event was diagnosed or unacceptable toxicity developed.
PARTICIPANTS
Women 35 years of age or older were eligible if they were postmenopausal (older than 50 years of age with no spontaneous menses for at least 12 months; or 50 years of age or younger either with no spontaneous menses [amenorrheic] within 12 months of randomization [e.g., spontaneous or secondary to hysterectomy] and a follicle-stimulating hormone level within the postmenopausal range or with prior bilateral oophorectomy). In addition, women had at least one of the following risk factors: age 60 years or older; Gail risk score greater than 1.66%; prior atypical ductal or lobular hyperplasia or lobular carcinoma in situ on breast biopsy or prior ductal carcinoma in situ treated with mastectomy. Prior menopausal hormone therapies (estrogen with or without progestin), luteinizing hormone–releasing hormone analogues, prolactin inhibitors, antiandrogens, or selective estrogen-receptor modulators were allowable, but not within 3 months of randomization. Women were ineligible if they were premenopausal, had prior invasive breast cancer or prior ductal carcinoma in situ treated with lumpectomy, were known carriers of the BRCA1 or BRCA2 gene, had a history of other malignancies (except nonmelanoma skin cancer, treated in situ cancer of the cervix, or other solid tumors treated with no evidence of disease for 5 years), had uncontrolled hypothyroidism or hyperthyroidism, or had chronic liver disease.
END POINTS AND ASSESSMENT
The primary outcome was incidence of invasive breast cancer. Secondary end points included a combined incidence of invasive and noninvasive (ductal carcinoma in situ) breast cancer; incidence of receptor-negative invasive breast cancer; incidence of combined atypical ductal hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ; number of clinical breast biopsies; clinical fractures; adverse cardiovascular events, including myocardial infarction or coronary heart disease that resulted in death; overall incidence of other cancers; the side-effect profile and safety; and health-related and menopause-specific qualities of life (assessed by means of the Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36] and the Menopause-Specific Quality of Life [MENQOL] questionnaire, respectively34-36).
At baseline, each patient’s history of prior diseases and treatment, family history of cancer, and reproductive history were obtained. Physical examination was performed, including height, weight, blood pressure, pulse, and clinical breast examination to confirm no suspicious breast abnormalities. Other requirements included complete blood count, liver-function tests, renal-function tests, and normal results on bilateral mammography and bone-mineral-density measurements within the past year. Symptoms at baseline were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.37 Quality of life (QOL) was measured within 1 week before randomization. Clinical assessments occurred at 6 and 12 months after randomization and yearly thereafter and included physical examination with clinical breast examinations, recording of concomitant medications, symptoms and adverse events, and QOL assessments. Women discontinuing the study drug continued to undergo clinical assessments and follow-up for clinical outcomes and adverse events.
Mammography was required within 12 months before randomization and every 12 months from the time of the initial mammogram during and after the treatment. Breast cancers could be detected on clinical breast examination during the clinic visits or on annual mammography. All mammograms and radiographic reports of fractures were reviewed centrally, and all evidence of disease on breast-biopsy specimens was reviewed by an adjudication committee. Study accrual and safety data were reviewed every 6 months by an independent data and safety monitoring committee. The original protocol and subsequent amendments are available at NEJM.org.
STATISTICAL ANALYSIS
The planned final analyses presented here include invasive breast cancer incidence and other secondary breast cancer end points, estimated on the basis of time from randomization to when an end point was reached. The sample-size estimate was based on an assumption of a rate of invasive breast cancer of 0.60% per year in the group given placebo, as compared with 0.21% in those treated with exemestane, with a relative reduction of 65% with exemestane. To detect this with a two-sided 5% level and 90% power, a total of 38 cases of invasive breast cancer were required, projected to occur when 4560 women were randomly assigned to treatment groups in a 3-year period and then followed for an additional 1.2 years. No interim analyses were planned. Accrual was completed on March 23, 2010; the protocol target-event rate was met on November 5, 2010. All data queries were resolved, and the database was locked on March 1, 2011.
Comparisons of time-to-event primary and secondary end points were based on the stratified log-rank test, adjusting for the two stratification factors at randomization. Cox proportional-hazards models were used to derive hazard ratios and associated 95% confidence intervals. Fisher’s exact test was used to compare adverse events between the treatment and placebo groups. Mean change scores from baseline to each assessment were calculated for all SF-36 and MENQOL subscales and summaries. Changes measuring 5 to 10% of the scale breadth or 0.5 SD were considered to be potentially clinically meaningful.38-40 Scores measuring changes in QOL were considered worsened if they decreased by 5 or more points (out of a total of 100) from baseline on the SF-36 and if they increased by 0.5 or more points (out of a total of 8) on the MENQOL. A chi-square test was used to compare the differences in proportions of patients found to have potentially clinically meaningful changes in QOL.
The study drug, exemestane, and funding support were provided by Pfizer, but this sponsor had no role in the design of the study or in the accrual, management, or analysis of the data. The decision to publish and the drafting of the manuscript were undertaken entirely by the first author, coauthors, and staff at the NCIC CTG central office, who vouch for the fidelity of the study to the protocol and for the accuracy and completeness of the data.
Results
Between February 11, 2004, and March 23, 2010, 4560 women were randomly assigned to either exemestane (2285 patients) or placebo (2275 patients). After randomization, 15 women (6 taking exemestane and 9 taking placebo) were considered to be ineligible to continue with the study but are included in the primary intention-to-treat analysis as randomly assigned. The exemestane and placebo groups were well balanced for race, body-mass index, and breast cancer risk factors (Table 1, and Table 1 in the Supplementary Appendix).
Table 1. Baseline Characteristics of Patients Randomly Assigned to Exemestane or Placebo.
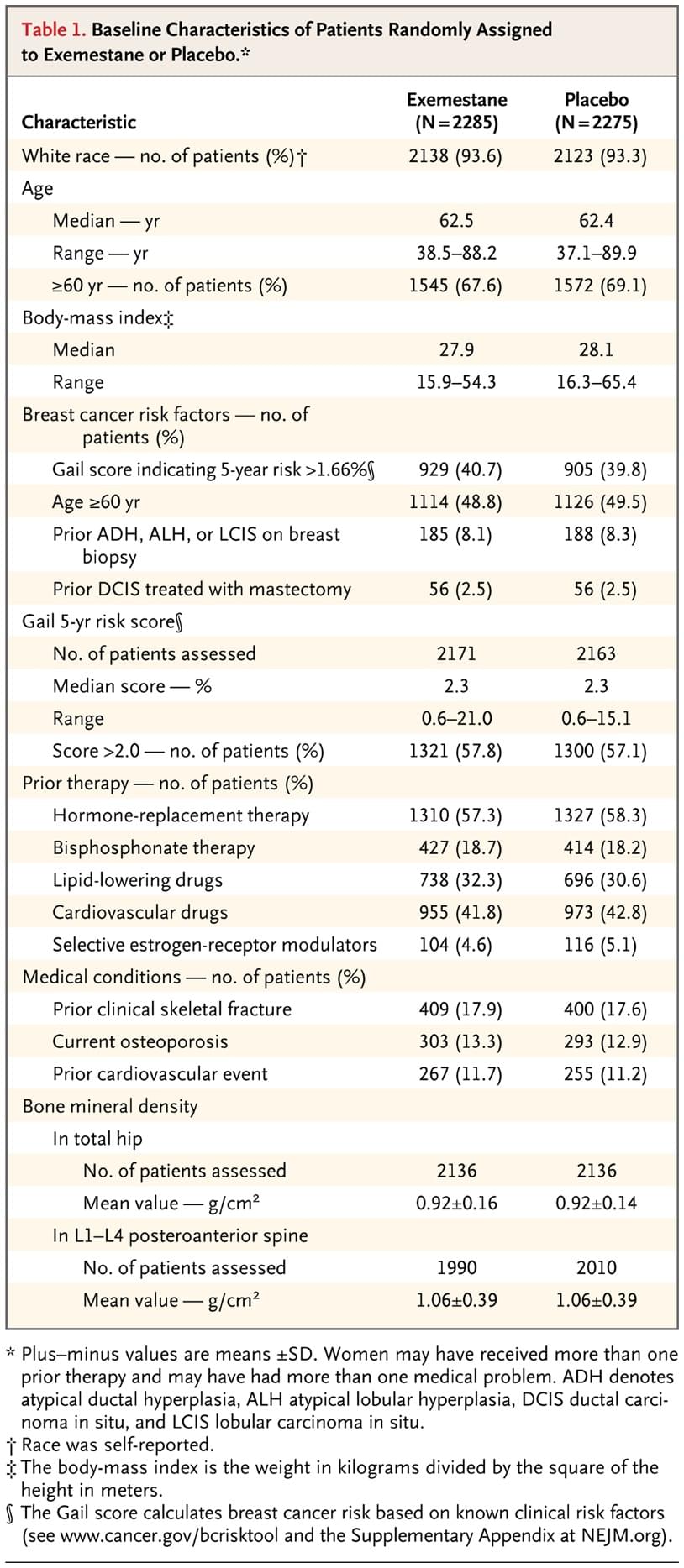
Major risk factors among the women who were enrolled included age of at least 60 years (49%); 5-year risk of breast cancer developing (Gail risk score >1.66%) (40%), and prior atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, or prior ductal carcinoma in situ treated with mastectomy (11%). Prior menopausal hormone therapy use was recorded in 1310 women in the exemestane group (57.3%; range from 1 to 588 months) and 1327 women in the placebo group (58.3%; range from 1 to 360 months). Pretreatment bone mineral density and prior history of clinical fractures, cardiovascular risk factors, and the concomitant use of bisphosphonates, lipid-lowering drugs, and cardiovascular drugs were similar in the two study groups.
On November 5, 2010, at the time of the clinical data cutoff, 735 women (32.8%) assigned to exemestane and 646 women (28.7%) assigned to placebo were no longer taking the study medication. About 5% in each group had discontinued the protocol treatment because of treatment completion. The major reasons for early discontinuation of the protocol treatments were toxic effects (15.4% in the exemestane groups vs. 10.8% in the placebo group, P<0.001) and patient refusal (6.9% vs. 6.0%, P=0.22). The median time from randomization to off-protocol treatment was 10.2 months (range, 0.1 to 61.5) for exemestane and 14.2 months (range, 0.1 to 62.9) for placebo. Approximately 85% of women were compliant and 15% were noncompliant with the protocol guidelines for the study treatments. Scheduled annual mammography was performed equally in the two groups, with 7.2% and 7.7% of women having missed at least one scheduled mammography appointment in the exemestane and placebo groups, respectively.
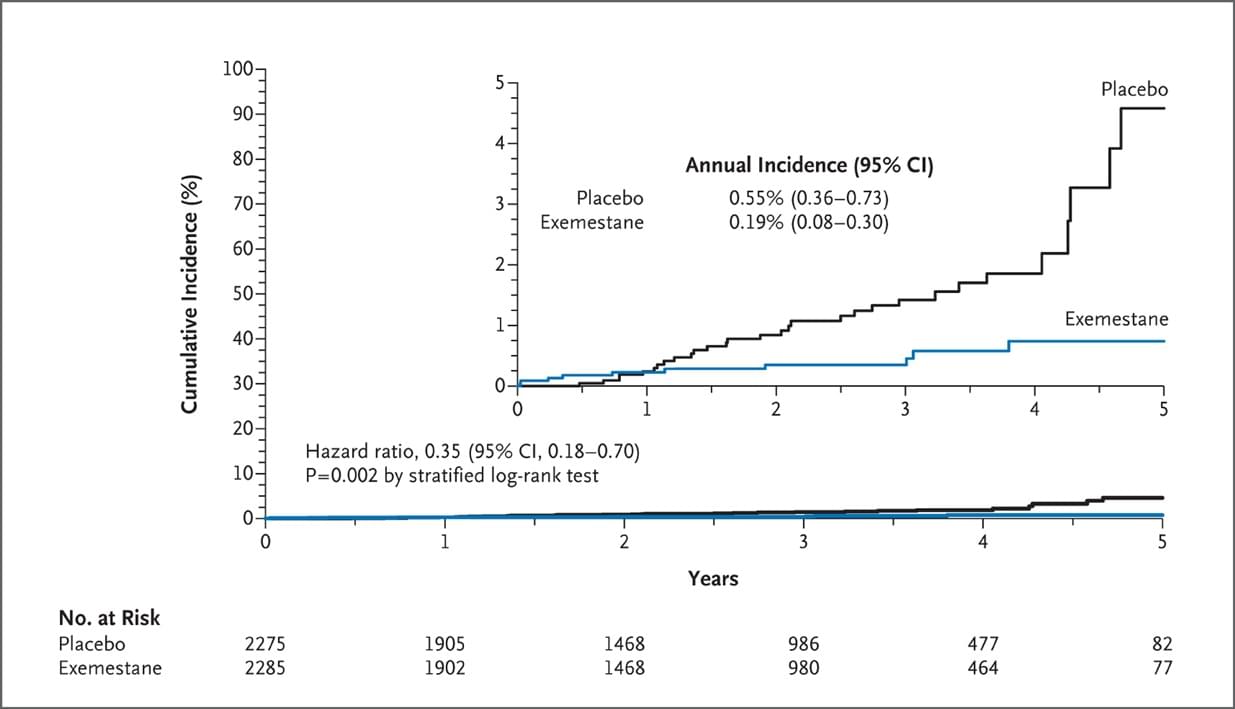
At a median of 35 months of follow-up (range, 0 to 63.4), 43 invasive breast cancers were diagnosed: 11 in the exemestane groups and 32 in the placebo group (annual incidence, 0.19% with exemestane vs. 0.55% with placebo; hazard ratio, 0.35 with exemestane; 95% confidence interval [CI], 0.18 to 0.70) (Table 2). Figure 1 shows the cumulative incidence of invasive breast cancer in these two groups. There were 37 ductal (10 in the exemestane groups and 27 in the placebo group) and 6 lobular (1 in the exemestane groups and 5 in the placebo group) cancers. The majority of cancers in each group were estrogen-receptor–positive, HER2/neu–negative, and node-negative (Table 2).
Table 2. Incidence of Invasive and Preinvasive Breast Events by Treatment Group.
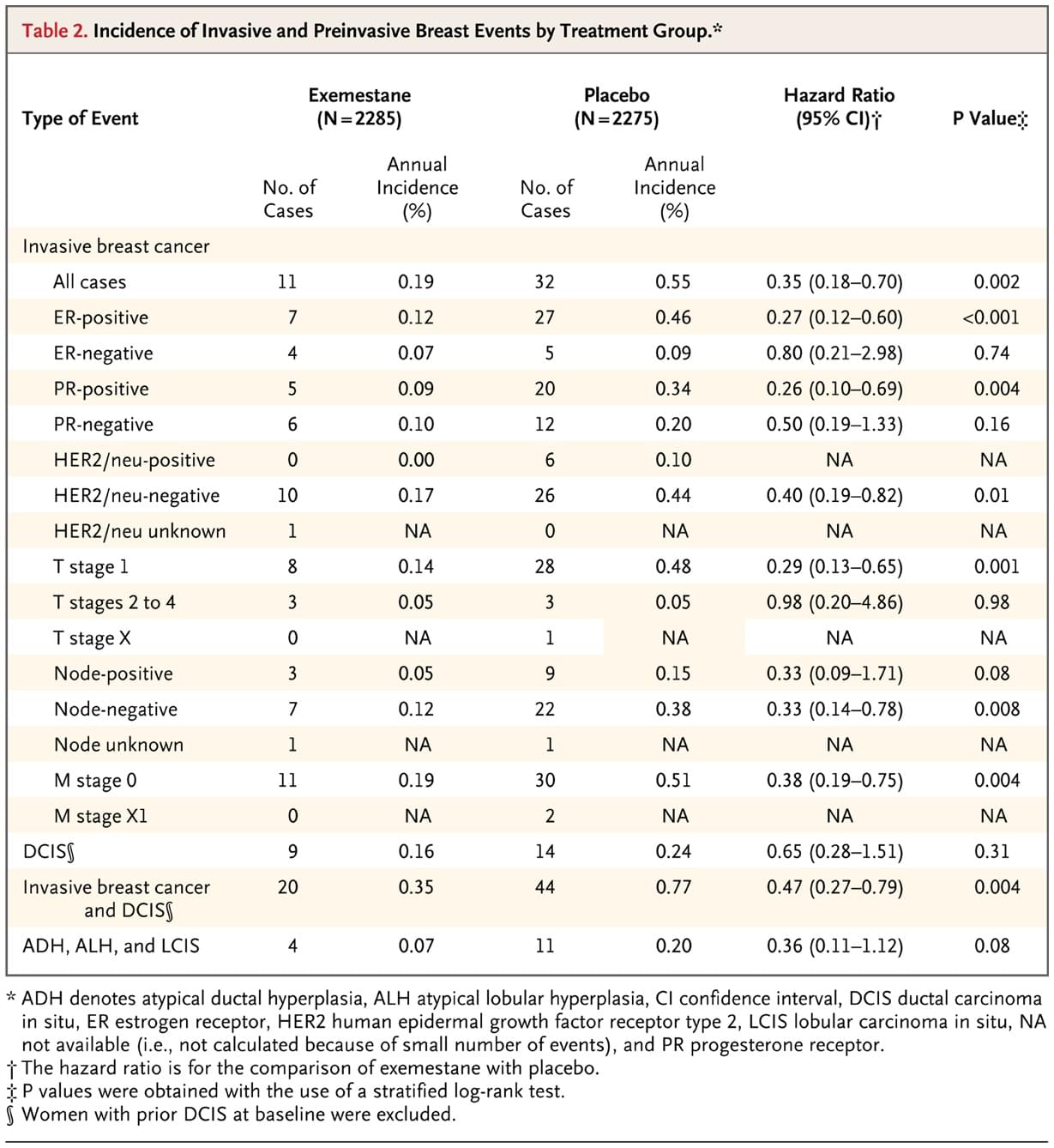
Figure 1. Cumulative Incidence of Invasive Breast Cancer.
Exemestane appeared to be superior to placebo in all prespecified subgroups defined by concurrent use of low-dose aspirin, Gail risk score, age, body-mass index, prior atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ and prior ductal carcinoma in situ treated with mastectomy (Figure 2). Exemestane also appeared to be superior in unplanned subgroups: invasive breast cancers according to prior use of menopausal hormone therapy (hazard ratio, 0.30 for prior users; 95% CI, 0.11 to 0.81; hazard ratio, 0.41 for prior nonusers; 95% CI, 0.16 to 1.05) and continent of residence (hazard ratio, 0.34 for North America; 95% CI, 0.16 to 0.71; hazard ratio, 0.39 for Europe; 95% CI, 0.07 to 1.99). The annual incidence of invasive breast cancer plus ductal carcinoma in situ (20 in the exemestane group and 44 in the placebo group) was 0.35% and 0.77% in the exemestane and placebo groups, respectively (hazard ratio, 0.47; 95% CI, 0.27 to 0.79). Combined lobular carcinoma in situ, atypical ductal hyperplasia, and atypical lobular hyperplasia occurred in 4 women (0.2%) in the exemestane group and 11 (0.5%) in the placebo group (hazard ratio, 0.36; 95% CI, 0.11 to 1.12). The number needed to treat to prevent one case of invasive breast cancer with exemestane therapy was 94 in 3 years and 26 in 5 years, but few women completed 5 years of therapy.
Table 3. Side Effects during Treatment, According to Severity.
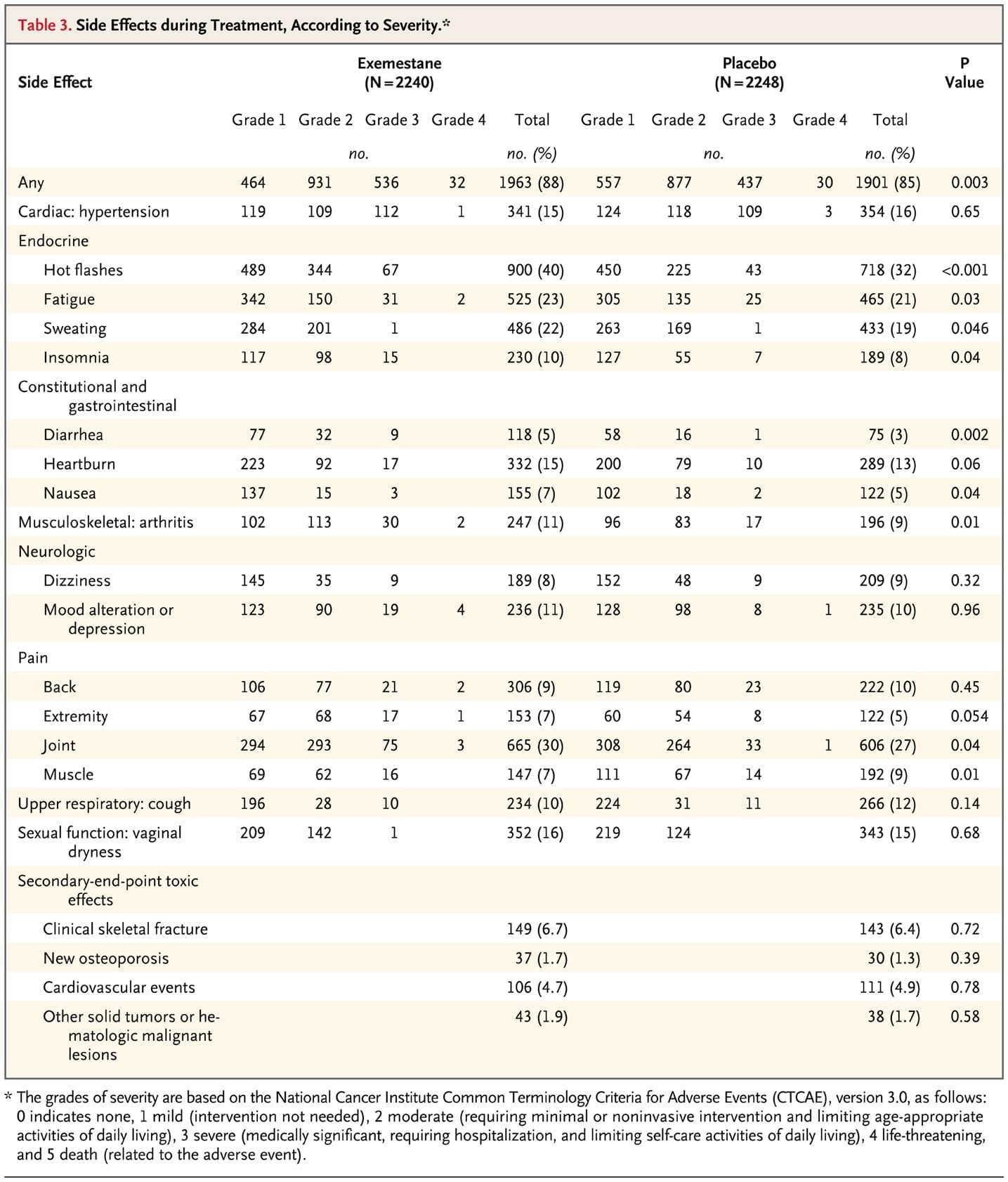
Table 3 (and Table 2 in the Supplementary Appendix) shows adverse events that occurred in 5% or more of women, with a difference between groups of 1% or more and prespecified secondary end points of toxicity. Symptoms and adverse events (all grades) occurred in 88% of women in the exemestane group versus 85% in the placebo group (P=0.003). Arthritis (P=0.01) and hot flashes (P<0.001) were more common in the exemestane group, but differences between the groups in the frequency of those with grade 2 or higher symptoms were modest (arthritis, 6.5% vs. 4.0%; hot flashes, 18.3% vs. 11.9%). Table 3 (and Table 2 in the Supplementary Appendix) shows no significant differences between the two groups in prespecified secondary end points, including new diagnoses of osteoporosis or cardiovascular events. Clinical fracture rates were also similar in the two groups, and the proportion of women in each group who were prescribed bisphosphonate therapy during the trial was also similar (24.5% for exemestane and 24.1% for placebo). There was no significant difference in the number of cancers other than breast cancer (50 [2.2%] vs. 44 [2.0%]) or time to detection of these cancers (1.8 yr vs. 1.6 yr). No significant differences were detected between the two groups with respect to hypercholesterolemia, hypertriglyceridemia, abnormal liver-function tests, acne, alopecia, rash, weight gain, or hair loss (data not shown). Table 3 in the Supplementary Appendix shows health-related and menopause-specific QOL results. Compliance in completing the QOL questionnaire at each follow-up visit was 92.9 to 97.4% for the exemestane group and 94.3 to 97.5% for the placebo group. No between-group differences in overall health-related QOL responses were found when distributions of worsened, stable, and improved scores on the SF-36 (Physical and Mental Component Scores) were compared despite worsened menopause-specific QOL among those taking exemestane (7% more overall). There were 38 deaths during the study (19 in each group). Causes of death in the exemestane and placebo groups, respectively, were breast cancer, 1 and 0; other malignancies, 10 and 12; cardiovascular events, 5 and 4; and other causes, 3 and 3. None were adjudicated as treatment-related.
Discussion
In this randomized, placebo-controlled trial in healthy postmenopausal women, exemestane reduced the relative incidence of invasive breast cancers by 65%, from 0.55% to 0.19%. Exemestane also reduced the risk of known breast-cancer precursor lesions — ductal carcinoma in situ, lobular carcinoma in situ, atypical ductal hyperplasia, and atypical lobular hyperplasia — suggesting possible further reductions in invasive cancers during long-term follow-up. Most tumors in these study patients were estrogen-receptor–positive. HER2-positive tumors, which have a poor prognosis, were also reduced with exemestane. Future studies to corroborate this finding would be important.
Menopausal symptoms such as hot flashes, fatigue, sweating, insomnia, and arthralgia were frequent among all the women in the study but were predictably somewhat more common in those taking exemestane. Also of potential clinical importance, more women in the exemestane group self-reported that menopause-related vasomotor and sexual symptoms had worsened. However, these symptoms did not appear to affect self-reports of overall health-related QOL among those taking exemestane because summary measures of physical and mental components of the SF-36 did not differ between the two study groups. (Full QOL results are not reported here.) Unlike the rare endometrial cancers and thromboemboli associated with tamoxifen, particularly in postmenopausal women, no serious adverse events or end-organ toxic effects, including fractures, were attributable to exemestane. Mild loss of bone mineral density with the aromatase inhibitors is well documented, but the annual excess incidence of fractures in trials comparing aromatase inhibitors and tamoxifen are probably due mainly to the bone-protective effects of tamoxifen.41,42 After the cessation of therapy in several large trials comparing aromatase inhibitors and tamoxifen in early breast cancer, bone mineral density improved and the difference in fracture rates was reduced.43-45 In these trials, adverse events also attenuated rapidly after cessation of treatment and correlated with recovery of estrogen levels to their normal postmenopausal range. The absence of excess clinical fractures in patients treated with exemestane in this study is reassuring. This occurred despite similar baseline bone mineral density in the two groups and the use of bisphosphonate therapy both before and during the study. Although differences in the occurrence of colorectal, lung, and endometrial cancers and malignant melanomas have been reported in tamoxifen comparator trials, no differences were seen in this trial or in the placebo-controlled MA.17 trial.26,46 Small numerical, but not significant, differences in the number of cardiovascular events have also been reported in trials comparing aromatase inhibitors with tamoxifen,22,23,46 with more events among the patients treated with aromatase inhibitors, and these differences may have been due to the slightly protective effect of tamoxifen, as suggested by Mouridsen et al.47 It is reassuring that when the aromatase inhibitors were compared with placebo, these differences were not seen either in this prevention trial or in our early breast cancer MA.17 trial.
This trial has some limitations. The median follow-up of 3 years is relatively short, and although consistent with our projections, the total number of breast events (66) was small. The optimal duration of endocrine therapy for breast-cancer prevention is not known, but in a previous placebo-controlled trial of early breast cancer, we found that prolonged aromatase-inhibitor therapy was associated with continued reductions in the incidence of contralateral breast cancers even after the aromatase inhibitor was discontinued.48 The number of women needed to treat in MAP.3 to prevent one case of breast cancer is 94 with 3 years of exemestane therapy, but is projected to be 26 at 5 years, although the number of women who received treatment for a full 5 years was low. By identifying subgroups of participants in the MAP.3 trial who would benefit most or who would be most vulnerable to toxic effects, one might be able to reduce the number needed to treat.
Despite these limitations we found a favorable risk-to-benefit ratio with a strong preventive effect of exemestane and, with a limited median follow-up of 3 years, an excellent safety profile across a spectrum of women at average to high risk for breast cancer. We reached our protocol-specified number of events for this final analysis; after unblinding, women taking the active drug will be offered exemestane to complete 5 years of therapy, and MAP.3 sites will have the option of offering 5 years of exemestane treatment to those initially assigned to placebo. We and others are conducting placebo-controlled trials in healthy women and patients with early breast cancer to evaluate prolonged aromatase-inhibitor therapy in postmenopausal women of similar age. The results of these ongoing trials should contribute to our understanding of the long-term efficacy and toxicity of aromatase inhibitors.
Supported by the Canadian Cancer Society Research Institute, the Canadian Institutes for Health Research, Pfizer, and the Avon Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
This article (10.1056/NEJMoa1103507) was published on June 4, 2011, and updated on June 23, 2011, at NEJM.org.
We thank the 4560 women (2824 from the United States, 1285 from Canada, 432 from Spain, and 19 from France) who agreed to participate in this study; the trial committee; the many investigators, pharmacists, and clinical research associates involved in the trial; Drs. Joe Pater and Lois Shepherd, Dianne Johnston, and Andrea Hiltz for their enthusiastic and unwavering support; the members of the NCIC CTG Data Safety Monitoring Committee; the Central Office staff of the NCIC CTG who contributed to the conduct of the trial; and Pfizer Pharmaceuticals for support and for providing exemestane and placebo.
Author Affiliations
From Massachusetts General Hospital Cancer Center (P.E.G.) and Dana–Farber Cancer Institute (J.E.G.) — both in Boston; Mayo Clinic, Rochester, MN (J.N.I.); Hospital Nuestra Señora De Sonsoles, Ávila, Spain (J.E.A., on behalf of the Spanish Group for Breast Cancer Research); Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, CA (R.T.C.); University at Buffalo, Buffalo, NY (J.W.-W.); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle (A.M.); University of California, Davis, Sacramento (J.R.); University of Tennessee Health Science Center, Memphis (K.C.J.); George Washington University School of Medicine, Washington, DC (L.W.M.); University of Wisconsin School of Medicine and Public Health, Madison (G.E.S.); Kansas University Medical Center, Kansas City (C.J.F.); Centre Hospitalier Universitaire Arnaud de Villeneuve, Montpellier, France (P.P., on behalf of the National Federation of French Cancer Centers); University Health Network, Toronto (A.M.C.), London Health Sciences Centre, London, ON (E.W.), Unité de recherche en santé des populations de l’Université Laval, Quebec (E.M.), Queen’s University Pathology and Molecular Medicine, Kingston, ON (P.F.), British Columbia Cancer Agency, Vancouver, BC (K.A.G.), and NCIC Clinical Trials Group, Kingston, ON (D.T., H.R.) — all in Canada.
Address reprint requests to Dr. Goss at Massachusetts General Hospital Cancer Center, Lawrence House, LRH-302, Boston, MA 02114, or at [email protected].
The NCIC Clinical Trials Group MAP.3 (NCIC CTG MAP.3) investigators are listed in the Supplementary Appendix, available at NEJM.org.
References
1. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med 2001;344:276-285[Erratum, N Engl J Med 2001;344:1804.]
2.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008;17:2029-2035
3.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TIA. Growth patterns and the risk of breast cancer in women. N Engl J Med 2004;351:1619-1626
4.The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606-616
5.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-1388
6.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003;361:296-300
7.Cuzick J. Long-term follow-up in cancer prevention trials (It ain’t over till it’s over). Cancer Prev Res (Phila) 2010;3:689-691
8.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006;355:125-137
9.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004;96:1751-1761
10.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727-2741
11.Armstrong K, Quistberg DA, Micco E, Domchek S, Guerra C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch Intern Med 2006;166:2260-2265
12.Lippman SM. The dilemma and promise of cancer chemoprevention. Nat Clin Pract Oncol 2006;3:523-523
13.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 2010;28:3090-3095
14.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology 2008 clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol 2009;27:3235-3258
15.Waters WA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev 2010;19:443-446
16.Chlebowski RT, Col N, Winer EP, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol 2002;20:3328-3343
17.Gail MH. Personalized estimates of breast cancer risk in clinical practice and public health. Stat Med 2011;30:1090-1104
18.Goss PE. Breast cancer prevention — clinical trials strategies involving aromatase inhibitors. J Steroid Biochem Mol Biol 2003;86:487-493
19.Lubet RA, Steele VE, Casebolt TL, Eto I, Kelloff GJ, Grubbs CJ. Chemopreventive effects of the aromatase inhibitors vorozole (R-83842) and 4-hydroxyandrostenedione in the methylnitrosourea (MNU)-induced mammary tumor model in Sprague-Dawley rats. Carcinogenesis 1994;15:2775-2780
20.De Coster R, Van Ginckel RF, Callens MJ, Goeminne NK, Janssens BL. Antitumoral and endocrine effects of (+)-vorozole in rats bearing dimethylbenzanthracene-induced mammary tumors. Cancer Res 1992;52:1240-1244
21.Schieweck K, Bhatnagar AS, Batzl C, Lang M. Anti-tumor and endocrine effects of non-steroidal aromatase inhibitors on estrogen-dependent rat mammary tumors. J Steroid Biochem Mol Biol 1993;44:633-636
22.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003;98:1802-1810
23.The Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer.N Engl J Med 2005;353:2747-57. [Erratum, N Engl J Med 2006;354:2200.]
24.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004;350:1081-1092[Erratum, 2004;351:2461, 2006;355:1746.]
25.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomized phase 3 trial. Lancet 2011;377:321-331
26.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793-1802
27.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010;28:509-518
28.Goss PE, Qi S, Josse RG, et al. The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone 2004;34:384-392
29.Joahannessen DC, Engan T, Di Salle E, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res 1997;3:1101-1108
30.Goss PE, Hadji P, Subar M, Abreu P, Thomsen T, Banke-Bochita J. Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Res 2007;9:R52-R52
31.Tu D. Minimization Procedure. In: Chow SC, ed. Encyclopedia of biopharmaceutical statistics. 3rd ed. New York: Marcel Dekker, 2010:795-8.
32.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879-1886
33.Solomon SD, McMurray JJV, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005;352:1071-1080
34.Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976;25:3130-3139
35.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996;24:161-175[Erratum, Maturitas 1996;25:231.]
36.Ware JE, Kosinksi M, Dewey JE. How to score version 2 of the SF-36 health survey. Lincoln, RI: QualityMetric, 2000.
37.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0. March 31, 2003.
38.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582-592
39.Wyrwich KW, Fihn SD, Tierney WM, Kroenke K, Babu AN, Wolinsky FD. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease: an expert consensus panel report. J Gen Intern Med 2003;18:196-202
40.Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 2005;23:6931-6940
41.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol 2006;24:3629-3635
42.Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-1057
43.Coleman RE, Banks LM, Girgis SI, et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat 2010;124:153-161
44.Geisler J, Lonning PE, krag LE, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer 2006;42:2968-2975
45.Eastell R, Adams J, Clack G, et al. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol 2011;22:857-862
46.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-1141
47.Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trial. J Clin Oncol 2007;25:5715-5722
48.Ingle JN, Tu D, Pater JL, et al. Duration of letrozole treatment and outcomes in the placebo-controlled NCIC CTG MA.17 extended adjuvant therapy trial. Breast Cancer Res Treat 2006;99:295-300
Source:
https://www.nejm.org/doi/full/10.1056/nejmoa1103507
