Zinc stearate
Zinc stearate is a “zinc soap” that is widely used industrially. In this context, soap is used in its formal sense, a metal “salt” of a fatty acid. It is a white solid that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons (e.g., benzene) and chlorinated hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect. Its main application areas are the plastics and rubber industry, where it is used as a releasing agent and lubricant which can be easily incorporated.
CONTACT US- Overview
- Product Description
- Safety
- Packaging & Delivery
- Shipping & Payment FAQ
- Environmental
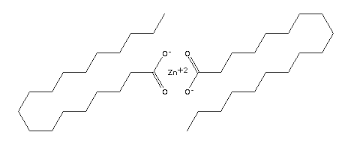
Synonyms: Afco-chem ZNS;ai3-00388 ;Antidust 2;caswellno926 ;Coad;Dermarone;Dibasic zinc stearate;dibasiczincstearate
CAS No: 557-05-1 (8028-87-3)
Molecular formula: C36H70O4Zn or Zn(C18H35O2)2
Molecular weight: 632.3 g/mol
Purity: N/A
Appearance: Soft, white powder
Melting point: 130 °C
Formulation: Zinc stearate USP; 60 g and 125 g shaker containers.
SMILES: CCCCCCCCCCCCCCCCCC(=O)[O-].CCCCCCCCCCCCCCCCCC(=O)[O-].[Zn+2]
InChi Key: AEMFNILZOJDQLW-QAGGRKNESA-N
Grade Standard: N/A
Brand name: Ikigai ™
Standard: USP, Ph. Eur., JP Etc
Storage: Zinc stearate is stable and should be stored in a cool, dry place
Shipping: N/A
Stability (shelf life): ≥ 1 year
Origin: PRC
| Category | Category Description | Categorization Type |
|---|---|---|
| Absorbent | Agent for soaking up liquid (product use or application unknown) | CPCat Cassette |
| Adhesive | General adhesives and binding agents for a variety of uses | CPCat Cassette |
| Adsorbent | Adhesion of molecules to a surface | CPCat Cassette |
| Antiadhesive | Used to prevent adhesion | CPCat Cassette |
| Antifriction | Additives to reduce friction | CPCat Cassette |
| Pictogram(s) |
  |
|---|---|
| Signal |
Warning
|
| GHS Hazard Statements | Aggregated GHS information provided by 1973 companies from 23 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria by 1179 of 1973 companies. For more detailed information, please visit ECHA C&L website Of the 20 notification(s) provided by 794 of 1973 companies with hazard statement code(s): H335 (75.31%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (81.99%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H413 (59.82%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
| Precautionary Statement Codes | P261, P271, P273, P304+P340, P312, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.) |
Disposal Methods
SRP: The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational harm/injury/toxicity or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal and plant life; and conformance with environmental and public health regulations.
FDA Requirements
The substances listed in paragraph (b) of this section may be safely used as antioxidants and/or stabilizers in polymers used in the manufacture of articles or components of articles intended for use in producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food, subject to the provisions of this section: (a) The quantity used shall not exceed the amount reasonably required to accomplish the intended technical effect. Zinc stearate is included on this list.
LD50 Rat ip 0.25 g/kg /SRP: 250mg/kg/
LD50 Rat oral >5000 mg/kg
LC50 Rat Inhalation >200 mg/L (1 hr)
LD50 Rabbit dermal >2000 mg/kg
Androstenedione’s production and administration as a drug to treat symptoms due to male andropause, dietary supplement, and athletic performance-enhancing drug may result in its release to the environment through various waste streams. Androstenedione is a naturally occurring androgen produced by the adrenal gland and converted to testosterone or estrone. It is produced naturally in humans during the production of testosterone and estrogen. It can be formed in soils via the metabolism of testosterone and estradiol occurring in poultry and livestock manures used as agricultural fertilizers. If released to air an extrapolated vapor pressure of 7.35X10-9 mm Hg at 20 °C indicates androstenedione will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase androstenedione will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals and with ozone; the half-lives for these reactions in the air are estimated to be 3.5 hours and 1 day respectively. Particulate-phase androstenedione will be removed from the atmosphere by wet and dry deposition. Androstenedione absorbs light at wavelengths >290 nm, and therefore, may be susceptible to direct photolysis. If released to soil, androstenedione is expected to have no mobility based upon a Koc of 6170. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry’s Law constant of 3.7X10-8 atm-cu m/mole. Androstenedione is not expected to volatilize from dry soil surfaces based upon its vapor pressure. Results of soil column studies using various soils and sands found that half-life of androstenedione ranged from 1.7 to 77 hours. If released into water, androstenedione is expected to adsorb to suspended solids and sediment based upon the Koc. Using OECD Guideline 301B (CO2 Evolution Test), androstenedione reached 79-95% degradation over a 28-day incubation period which classified the compound as readily biodegradable. Volatilization from water surfaces is not expected to be an important fate process based upon this compound’s estimated Henry’s Law constant. An estimated BCF of 30 suggests the potential for bioconcentration in aquatic organisms is low to moderate. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions. Androstenedione in aqueous solutions underwent direct photolysis half-lives ranging from 3.7 to 10.8 hours in direct sunlight. Occupational exposure to androstenedione may occur through dermal contact with this compound at workplaces where androstenedione is produced or used.
2000 Metric Ton/Metric Tons per Month
Packing:
Export worthy packing (1kg/Aluminium foil bag;25kg/drum) Or as per customers
request.
Ports: port of Hong Kong HKSAR
of PRC., port of Ningbo, PRC
or Any port in
China.
Delivery methods:
- by Air Freight & Cargo
- by Sea Freight & Cargo (Container shipping)
- by Rail Freight (train)
- by Road Freight
- by Intermodal freight transport
By couriers:
- SF Express
- STO Express
- DHL
- FEDEX
- EMS
- TNT
- UPS
- and almost any available courier service

Answer: Pro-forma invoice will be sent first after confirmation of the order, enclosed our payment information. Payment by T/T, Paypal, and others. All courier tracking numbers are provided upon shipping.
Answer: The MOQ for this API is 1kg.
Answer: We have shipping insurance. Our experienced team will do the customs documents, generally, it will have no trouble. And we will reship or refund for you if it is seized by our mistake.
Answer: Shipping lead time: About 2-5 days after payment confirmed. (Chinese holiday not included), We have long-term relations with shipping agents in main Chinese ports. Full service can be offered, including photos of every shipment, marks, and procedure of loading. General times are gor 1KG-100KG, Within 5-7 days by DHL, UPS, TNT, FEDEX, EMS Over 100KG. within 5-8days by air, 20-40days by sea. More information.
Answer: Different quantity has a different discount. Please feel free to contact us.
Answer: You can chat with us by Telegram, Email, Skype, Whatsapp, Facebook and other methods – just inquire, we will give a reply ASAP. Please feel free to send your quotations.
Answer: Our quality control will reduce the quality problem to near zero. If there is a real quality problem caused by us, we will send you free goods for replacement or refund your loss. We are competitive in API exporting, Pharmaceutical excipients, food supplements, dyestuff, veterinary API. Our team has 20 years of experience in this business. Good team for quality control, shipping, and documents.
U.S. funds preferably.
The negative impact of the production of pharmaceutical products on the natural environment is well known. However, this remains largely unregulated, meaning the extremely toxic impact it has on both animals and humans continues with no clear end in sight. As Ikigai Corporation Company Innovator, we are committed to expanding and improving our efforts to safeguard the environment. We accordingly established our environmental management system

Quality Management, Environmental Management and Occupational Safety
Policy
Ikigai Corporation Company is engaged in the production and distribution of
pharmaceutical substances and other chemical specialties. As a chemical and
pharmaceutical manufacturer, the Company is aware of the impacts of its activities on
the product quality, on the environment and the health of its employees, and undertakes
to control them, with the aim of constant improvement.
As standards for the implementation and maintenance of the Integrated Management System, the Company has chosen international standards ISO 9001 in the area of quality management (hereinafter called QMS), ISO 14001 for the management of environmental protection (hereinafter called EMS) and specification OHSAS 18000 for ensuring the occupational safety and health protection (hereinafter called SMS). The Integrated Management System applies to all fields of the Company’s activities.
Within this Policy, Ikigai Corporation Company undertakes to:
Constantly improve all of its activities
Executive commitment to continually improve energy efficiency across the entire
corporation, including clear processes and tracking systems to identify
opportunities
An empowered corporate energy director and energy team supported by sufficient human and
financial resources
A corporate energy policy that is accounted for at the top levels of the corporation
Aggressive, numeric energy goals that stretch performance targets to draw out creative
innovations for meeting them
Measurement and tracking of energy performance for all energy use, corporationwide,
including benchmarking facility performance nationally and globally with similar
companies, and a review system with accountability at all levels
Communication of the value of energy savings, importance of improving use of energy and
executive commitment by consistently recognizing accomplishments.
Abide by the relevant legislation and other regulations which apply to the Company or
which the Company has committed to observe; especially the requirements arisen from the
Act on Pharmaceuticals, the Decree on Good Manufacturing Practice and other guidelines
and directives and other requirements related to the occupational safety and the
environmental protection.
Constantly educate and train its employees and strongly encourage them to improve the
production quality, protect the environment and observe the occupational safety
principles; to design the training so as to motivate the employees performing their jobs
to prevent or reduce negative impacts of all activities on the environment.
Develop communication and cooperation with the public administration bodies,
professional public and other parties concerned with the environmental protection
issues. Ikigai Corporation Company is interested in holding open dialogue with the
employees and the public, in informing both internal and external stakeholders of the
impacts of all the Company’s activities on the environment and in responding to
justified suggestions and concerns.
The executive management expects that all the Company employees will accept an opinion
that observing the above mentioned principles and objectives is one of the most
fundamental duties of every employee in the Company.
Leave a Reply
Your email address will not be published. Required fields are marked *



